Our Research
Our aim is to develop a single breath test to diagnose five major gastrointestinal cancers; oesophageal, gastric, pancreatic, liver and colorectal.
The problem to address
Unmet need
Late diagnosis is a common feature of major gastrointestinal cancers. Early stages are commonly associated with non-specific symptoms, often similar to benign conditions. The National Institute for Health and Care Excellence (NICE) referral guidelines are age-dependent and mainly include red-flag symptoms, resulting in cancer diagnosis at an advanced stage. Currently, the cancer yield of urgent and non-urgent referral pathways is 4.4-5% and 0.1-1.7%, respectively.
Burden of gastrointestinal cancers
In the UK, 75,277 patients are diagnosed and 44,455 die annually. Diagnosing cancer at an advanced stage permits fewer curative treatment options, reducing chance of cure. Overall, 5-year survival for oesophageal, gastric, pancreatic, liver and colorectal cancer is poor (15.9%, 20.7%, 9.0%, 12.6% and 50.7%, respectively) and largely stage-dependent.
Proposed solution
Low cancer yields from gastrointestinal investigations results from the lack of an intermediate tests to streamline referrals, apart from faecal immunochemical test for colorectal cancer. Breath test is our proposed solution as a triage test to direct patients with non-specific symptoms to have specialised investigations.
Overview of our work
The Hanna Group laboratory
State-of-the-art dedicated laboratory suite for breath analysis in clinical studies (Imperial College London, ICL) with non-VOC emitting infrastructure and positive pressure ventilation, encompassing four laboratories dedicated to different stages of workflow and including cell culture and tissue processing facilities for mechanistic studies underpinning VOC production.
VOC methodology
We established a reliable platform for high-throughput breath analysis with excellent VOC detection limits, quantification, reproducibility and analytical recovery. We have a validated analytical method for TD-GC-TOF-MS that adheres to European Medicines Agency guidance with VOC quantification limit at 1.25ng/L. We have developed quality assurance and control procedures to provide a high level of confidence in the complete workflow from breath sampling to data generation using a bespoke Laboratory Information Management System for logging all recorded data with full traceability. The Hanna laboratory plans to gain ISO-17025 accreditation in 2025.
VOC data Artificial Intelligence (AI) analytical pipeline
We developed an AI platform, MSHub, to process and analyse GC-MS data from breath samples using molecular network analysis to identify VOC biomarkers that separate cancer from control and other cancer types. It enables analysis of an unlimited data volume in a reproducible way since the algorithm was designed to function without user involvement. MSHub operates using out-of-core processing, with no processing limit beyond data storage capacity, allowing rollout to sites where high-performance computing is unavailable.
Mechanisms of VOC production
We showed in an experimental human model that the origin of certain VOCs within exhaled breath is derived principally from the lungs, suggesting that VOCs travel in the systemic circulation. We confirmed the endogenous origin of VOCs from cancer and its associated environment.
Biomarker discovery and clinical studies
- Oesophageal and gastric cancers: We developed a cancer detection model with internal validation (n=210 patients), using SIFT-MS with an area under the receiver operator characteristic (AU-ROC) curve of 0.92. The biomarker panel was externally validated in a multicentre study (n=335, AU-ROC=0.85). We expanded the biomarker panel using TD-GC-MS for analytical validation (n=300, AU-ROC= 0.90).
- Colorectal cancer: COBRA study (n=1432) identified biomarkers for colorectal cancer, achieving AU-ROC=0.91 in symptomatic patients and AU-ROC=0.87 when including asymptomatic population.
- Pancreatic cancer: We identified biomarkers for pancreatic ductal adenocarcinoma (n=132, AU-ROC=0.90).
- Liver cancer: VOCAL study identified biomarkers for hepatocellular carcinoma (n= 154, AU-ROC=0.94).
Intellectual Property
Biomarkers are protected and owned by ICL. The Hanna Group are inventors of seven patents filed worldwide.
Human factors studies
We demonstrated breath testing at public events and conducted simulation studies and human factors experiments on different breath collection devices to examine usability and contamination levels. Stakeholders’ engagement concluded that best position for breath testing is a triage tool in primary care.
Health economic modelling
Economic modelling projected that a breath test-assisted referral pathway for suspected oesophagogastric cancer would cost the NHS £138M compared to £293M for the current referral pathway with an annual saving of £155M.
Breath testing in the clinical environment
We demonstrated breath testing acceptability in primary care (MAGIC, n=1002) and large-scale multicentre studies (COBRA, n=1432), exceeding recruitment targets. Almost all (99%) of patients consider breath test easy/ very easy to do.
Current clinical trials
ICL are conducting large scale clinical trials in oesophageal, gastric, pancreatic, liver and colorectal; cancers (about 30,000 patients over next 3-4 years). See section on clinical trials.
Current translational research
Tumour
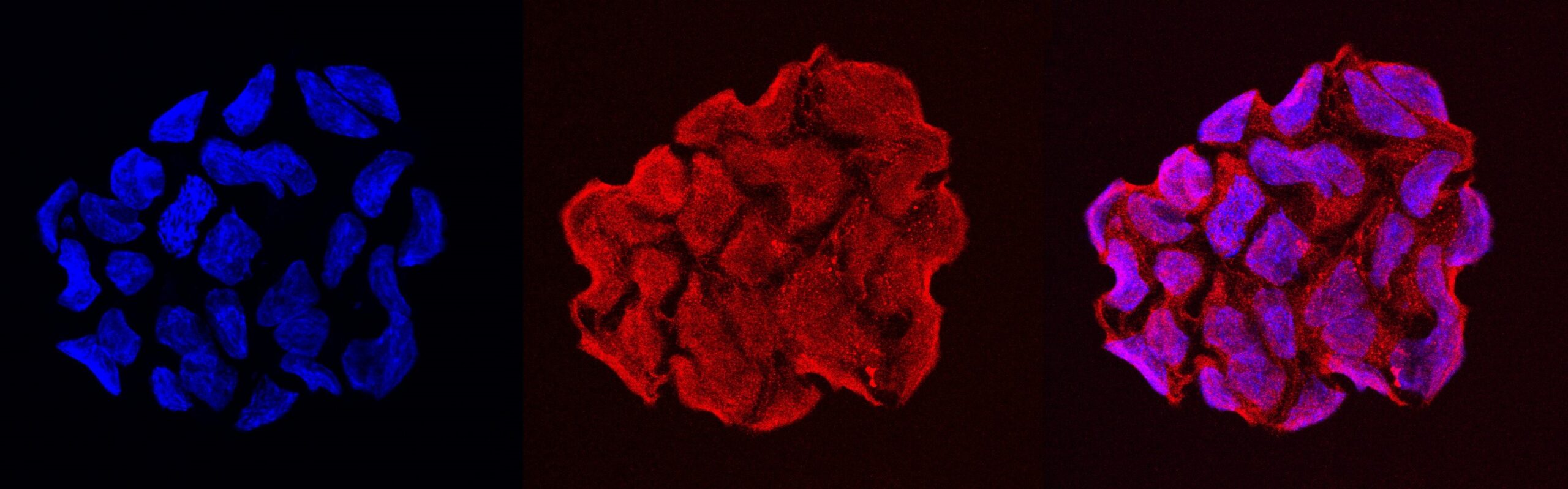
Regional hypoxia (low oxygen) and acidosis is a feature of all solid tumours, and is a consequence of rapid cellular proliferation and an inadequate blood supply. As a result, tumour cells undergo profound metabolic reprogramming to adapt to hypoxic, acidic and nutrient-deprived conditions. We speculate that these harsh conditions that are unique to the tumour microenvironment may play a role in the production of cancer-associated VOCs. Using a systems biology approach, we are investigating the influence of these factors on VOC production using cell & patient-derived organoid models. Experiments include pathway-directed genetic perturbation, functional assays to evaluate aggressive tumour biology. New clinical studies will also use state-of-the-art spatial -omic techniques and machine learning to bridge the gap between tumour metabolism and exhaled VOCs.
Microbiome
The human microbiome plays a significant role in homeostasis, immunomodulation and pathogenesis. The dynamic and intricate relationship between bacteria and fungi within the tumour microenvironment is critical in understanding factors influencing cancer development and progression. As a result, valuable predictive information can be gathered on diagnoses, prognostication and treatment strategies. We hypothesise there is a strong symbiotic or antagonist relationship between the cancer microbes which influences clinical outcomes. Our work will use high through-put next generation sequencing platforms to define the microbiome of oesophagogastric cancer, explore their functional capabilities and comprehensive network analyses to establish drivers of disease. Evaluating the underlying mechanistic pathways responsible for cancer metabolite production will further elucidate the origin of cancer-specific volatile organic compounds.
Immune
Tissue immune surveillance and infiltration impacts prognosis and therapy response in solid cancers. To better understand the influence of the host immune response on VOC generation and carcinogenesis in gastrointestinal cancers, we utilise high-dimensional techniques including spectral flow cytometry, imaging mass cytometry, and confocal microscopy, which allows us to comprehensively characterize heterogeneous immune cell populations at a single-cell level. This knowledge guides the design of bespoke tri-culture models incorporating patient-derived immune cells, organoids and fibroblasts to more accurately mimic the tumour microenvironment. By integrating immune cells into our VOC models, we can precisely replicate in vivo immune-tumour interactions, thereby enhancing our understanding of how various components of the tumour microenvironment contribute to VOC production. Furthermore, by combining microbial analysis and the use of fluorescence in situ hybridisation, we can investigate the microbial-immune interactions which may be responsible for initiating the spread of cancer and identify profiles associated with treatment response.
