COBRA
COlorectal BReath Analysis
Our funders




ClinicalTrials.gov Identifier: NCT05844514.
ClinicalTrials.gov Website:
https://clinicaltrials.gov/study/NCT05844514.
NIHR Clinical Research Network portfolio adoption:
CPMS ID: 34018.
Sponsor: Imperial College London.
Funding: National Institute for Health and Care Research.

Background
Colorectal cancer (CRC) is the second most common cause of cancer death in the United Kingdom, with approximately 17,000 deaths per year. A triage test to identify patients at risk of CRC could significantly improve survival and streamline referral pathways. Breath analysis offers a simple, quick and non-invasive method to detect volatile organic compounds (VOCs) in breath that are specific to CRC.
We carried out a discovery study (COBRA1) to identify potential VOC biomarkers to detect CRC in 855 symptomatic patients (709 controls, 146 patients with CRC). Fourteen VOC biomarkers for CRC were identified and we developed a detection model with an area under the receiver operating characteristic (AU-ROC) of 0.91, with a sensitivity of 83% and specificity of 88% [Gastroenterology. 2022, 163 (5), 1447-1449].
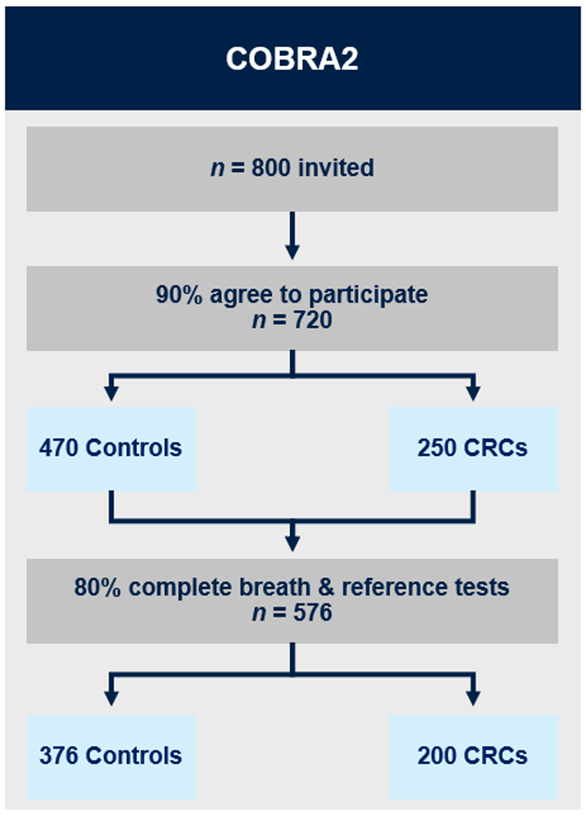
COBRA2 (currently recruiting)
Aim:
COBRA2 will validate VOCs for CRC previously identified in COBRA1 and discover further VOCs using enhanced analytical methods to refine the detection model. An exploratory comparison between the breath test and faecal immunochemical test will also be performed.
Methods:
COBRA2 is a prospective multicentre validation study. Breath testing will be performed in 720 patients (470 controls, 250 patients with CRC) attending hospital for a planned colonoscopy or for resection of a histologically confirmed colorectal adenocarcinoma. Breath will be collected on to thermal desorption (TD) tubes and analysed with mid-polar, polar and two-dimensional gas chromatography-time of flight mass-chromatography (GC-MS-TOF) in our laboratory.
Participating Centres:

Contact
For more information relating to the study, or if you are a healthcare professional interested in your centre participating in this study, please get in touch with Michael Fadel, NIHR Doctoral Fellow, via email: m.fadel@imperial.ac.uk.
